The HLB of sucrose esters
Sucrose esters are non ionic emulsifiers used as food additives (E473) and in cosmetics. They are obtained by grafting fatty acid chains (the hydrophobic part) on sucrose (the hydrophilic part), through an ester bond. As a result, they are amphiphilic compounds and are surface active (surfactants).
Sucrose has 8 hydroxyles functions. In industrial methods, all of them react with the fatty acid reagent (some of them react preferentially, especially the primary hydroxyles 1’, 6, 6’). As a result, depending on how much fatty acid reagent is used relative to sucrose, one, two, three…etc… up to eight fatty chains can be grafted on sucrose.
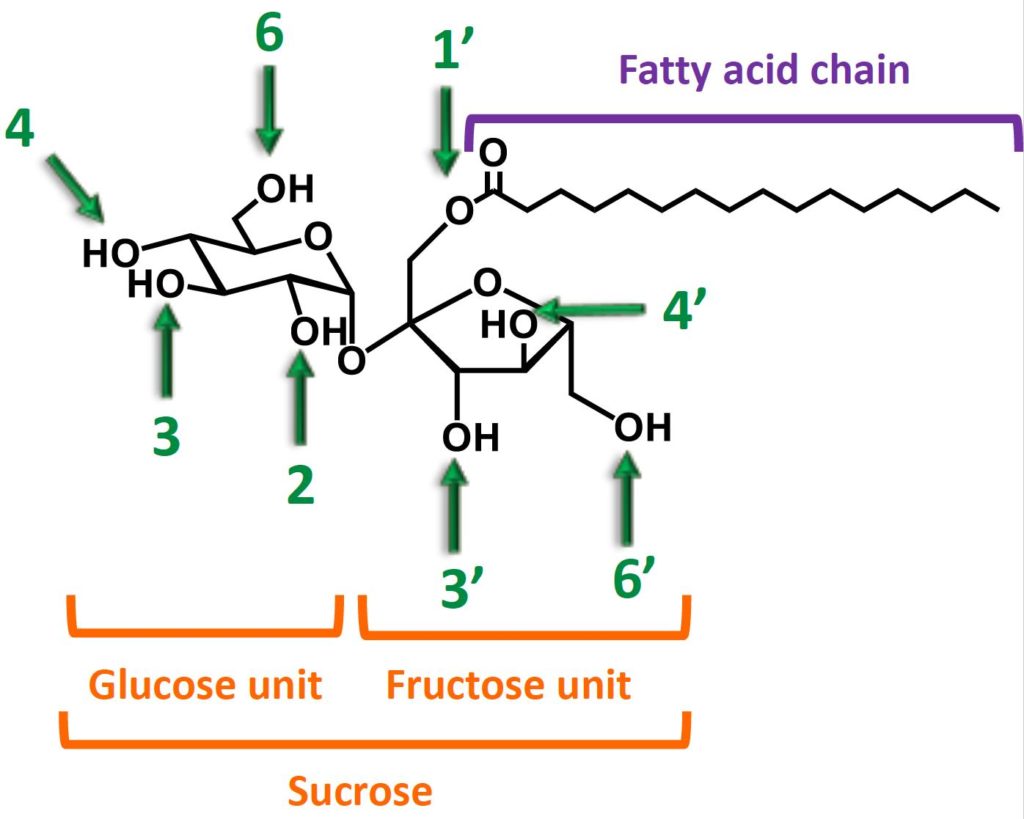
Exemple of sucrose monoester
Name:
- 1’-O-palmitoyl sucrose
- 1’-O-palmitoyl-α-D-glucopyranosyl-(1->2)-β-D-fructofuranoside
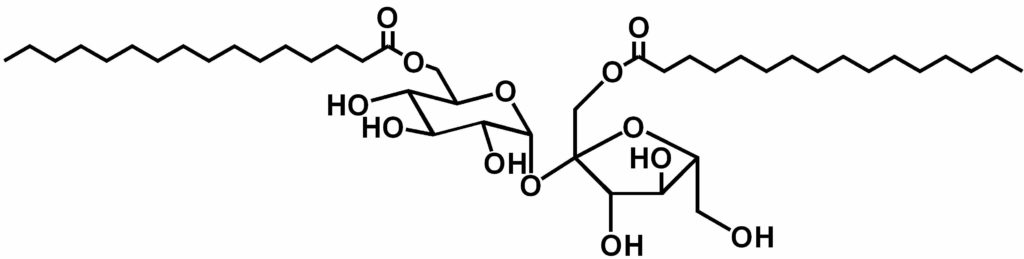
Exemple of sucrose diester
Name:
- 1’,6-di-O-palmitoyl sucrose
- 1’,6-di-O-palmitoyl-α-D-glucopyranosyl-(1->2)-β-D-fructofuranoside
As a result, industrial sucrose esters emulsifiers are blends of mono, di, tri, tetra…and more substituted esters of sucrose, in different proportions depending on the grade. The fatty chains are also grafted on different positions of sucrose.
When the fatty chain is shorter, the emulsifier blend is more hydrophilic. For example, sucrose laurates (12 carbons in the fatty chain) are more hydrophilic than sucrose myristoates (14 carbons), than sucrose palmitates (16 carbons), than sucrose stearates (18 carbons).
When the proportion of monoester in the blend is higher, the ratio of lipophilic fatty chain is lower, then the emulsifier blend is more hydrophilic. This ratio between the hydrophilic part and the hydrophobic (lipophilic) part of a surfactant is known as the HLB “Hydrophile Lipophile Balance”. In the case of sucrose esters blends, the HLB has been defined by calculation, by transposing the Griffin’s HLB scale established for an other family of non-ionic surfactants, PEO (polyethylene oxide) surfactants.
HLB scale
Griffin’s scale for PEO surfactants:
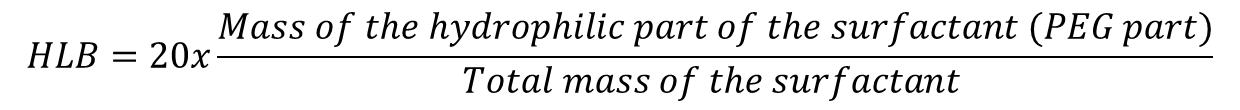
Transposition of the Griffin’s scale to sucrose esters:
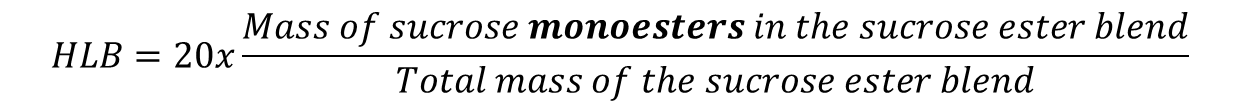
For example, a sucrose ester mixture containing 80% of sucrose monoester has a calculated HLB = 16, regardless the fatty acid chain length. The “calculated HLB” range usually defined by suppliers extend from 2 (for the most lipophilic blends) to 16 (for the most hydrophilic blends) depending on the sucrose ester grades.
Because this scale does not take into account the difference due to the chain length and because it does not relate to experimental data, this calculated HLB should be merely considered as an index ranking them from the most hydrophilic to the most lipophilic. It is useful for comparing their properties within the sucrose ester family, but it should not be used as an experimental predicting tool for comparing their emulsifying properties to other kinds of surfactants (such as PEO surfactants and others). Few scientific publications now tend to show that experimentally, the most hydrophilic sucrose esters blends, such as sucrose monolaurates or sucrose monopalmitates (both ranked with a HLB 16 by the suppliers) would have a behavior comparable to surfactants with HLB around 12 and 11 respectively.
Publications
- Wikipedia, article “Sucrose esters”, section “hydrophilic-lipophilic balance”
- Muller, A.-S.; Fitremann-Gagnaire, J.; Queneau, Y.; Karaoglanian, M.; Maitre, J.-P.; Bouchu, A. Winsor Behaviour of Sucrose Fatty Acid Esters: Choice of the Cosurfactant and Effect of the Surfactant Composition. Colloids and Surfaces A: Physicochemical and Engineering Aspects 2002, 203 (1), 55–66.
- Ontiveros, J. F.; Pierlot, C.; Catté, M.; Molinier, V.; Salager, J.-L.; Aubry, J.-M. A Simple Method to Assess the Hydrophilic Lipophilic Balance of Food and Cosmetic Surfactants Using the Phase Inversion Temperature of C10E4/n-Octane/Water Emulsions. Colloids and Surfaces A: Physicochemical and Engineering Aspects 2014, 458, 32–39.
