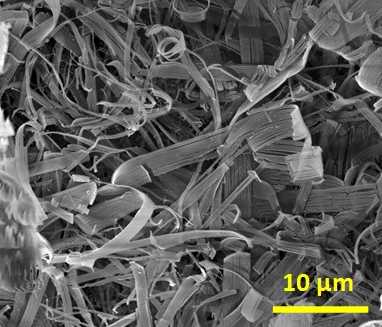
Molecular Gels Structure
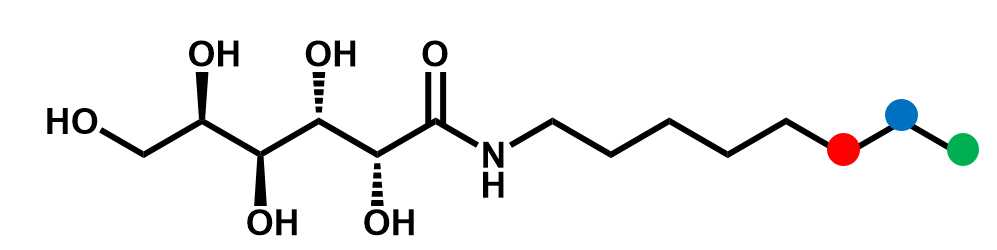
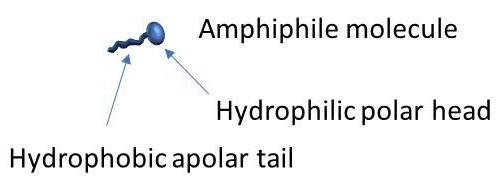
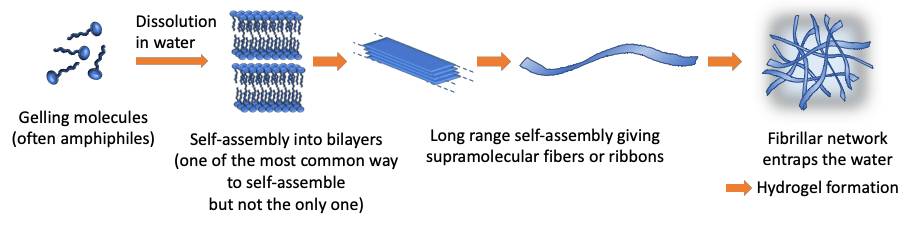
Molecular gels are formed by the self-assembly of low molecular weight molecules, namely «small», non-polymer molecules, in organic solvents or in water. They are polymer-free hydrogels. Many of them are amphiphilic molecules and one of the most common self-assembly mechanism of these molecules in water is displayed below.
SOME NOTES
- Molecular gels that are formed in water are called molecular hydrogels. Molecular gels that are formed in organic solvents are called molecular organogels.
- Many small molecules with various chemical structures (often amphiphilic) have been shown to gelate solvents (most often) and water (less often). The concentration at which gelation occurs is highly dependent on the gelator. Gelation typically occurs when the gelator concentration in the solvent (water or organic solvent) is between 0.1 wt% and 20wt%.
- Alternative denominations are:
- supramolecular gels (this denomination is also used for designing gels based on polymers in which the formation of the gel network (the cross-linking) relies on supramolecular non-covalent interactions instead of covalent interactions. So the denomination «molecular gel» is more dedicated for designing gels based on non-polymer molecules.
- Low-Molecular Weight Gels (LMWG)
- Low Molecular Mass Organic Gelators (LMOG). This acronyme also covers the concept of Low Molecular weight Organogels, the latter being restricted to the gelation of organic solvents (not water) by these “small” molecules.
- Self-Assembled Fibrillar Networks (SAFIN, this denomination includes gelling and non-gelling fibrillar networks derived from the self-assembly of small molecules)
To know more
- Wet Spinning of a Library of Carbohydrate Low Molecular Weight Gels. Bordignon, D.; Lonetti, B.; Coudret, C.; Roblin, P.; Joseph, P.; Malaquin, L.; Chalard, A.; Fitremann, J. Journal of Colloid and Interface Science 2021, 603, 333–343.
- Molecular Gels: Materials with Self-Assembled Fibrillar Networks; Weiss, R. G., Terech, P., Eds.; Springer Netherlands, 2006.
- Molecular Gels: Structure and Dynamics; Weiss, R. G., Ed.; Monographs in Supramolecular Chemistry; Royal Society of Chemistry: Cambridge, 2018.
- Low-Molecular-Weight Gels: The State of the Art, Draper, E. R.; Adams, D. J. Chem 2017, 3 (3), 390–410.
- Supramolecular Biomaterials. Webber, M. J.; Appel, E. A.; Meijer, E. W.; Langer, R. Nature Mater 2016, 15 (1), 13–26.
- Supramolecular Hydrogelators and Hydrogels: From Soft Matter to Molecular Biomaterials, Du, X.; Zhou, J.; Shi, J.; Xu, B. Chem. Rev. 2015, 115 (24), 13165–13307.
- Insights into Low Molecular Mass Organic Gelators: A Focus on Drug Delivery and Tissue Engineering Applications. Skilling, K. J.; Citossi, F.; Bradshaw, T. D.; Ashford, M.; Kellam, B.; Marlow, M. Soft Matter 2013, 10 (2), 237–256.
- Low molecular-mass organic gelators: Wikipedia
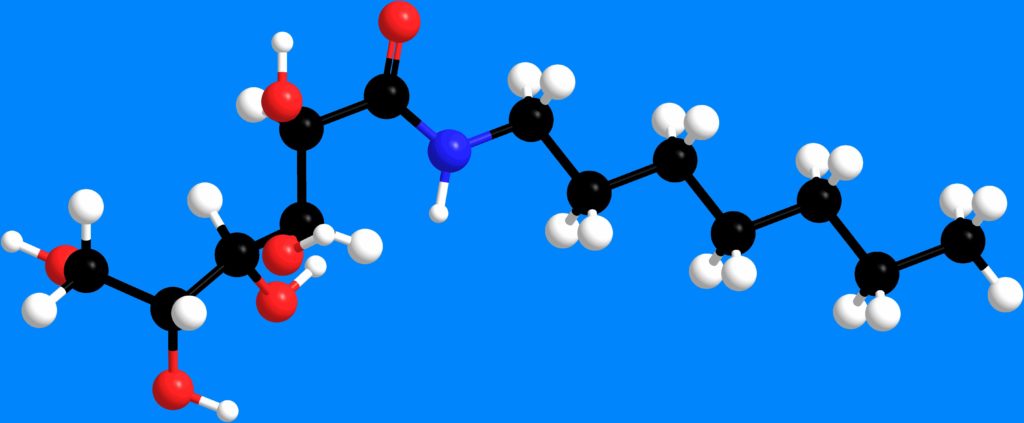
N-alkyl-D-galactonamide hydrogels
Some molecular hydrogels are based on very simple sugar-based molecules, such as N-alkyl-D-galactonamides
Molecular model of N-heptyl-D-galactonamide
Publications
- Chalard, A.; Vaysse, L.; Joseph, P.; Malaquin, L.; Souleille, S.; Lonetti, B.; Sol, J.-C.; Loubinoux, I.; Fitremann, J. Simple Synthetic Molecular Hydrogels from Self-Assembling Alkylgalactonamides as Scaffold for 3D Neuronal Cell Growth. ACS Appl. Mater. Interfaces 2018, 10 (20), 17004–17017.
- Chalard, A.; Joseph, P.; Souleille, S.; Lonetti, B.; Saffon-Merceron, N.; Loubinoux, I.; Vaysse, L.; Malaquin, L.; Fitremann, J. Wet Spinning and Radial Self-Assembly of a Carbohydrate Low Molecular Weight Gelator into Well Organized Hydrogel Filaments. Nanoscale 2019, 11 (32), 15043–15056.
- Chalard, A.; Mauduit, M.; Souleille, S.; Joseph, P.; Malaquin, L.; Fitremann, J. 3D Printing of a Biocompatible Low Molecular Weight Supramolecular Hydrogel by Dimethylsulfoxide Water Solvent Exchange. Additive Manufacturing 2020, 33, 101162.
- Pfannemüller, B.; Welte, W. Amphiphilic Properties of Synthetic Glycolipids Based on Amide Linkages. I. Electron Microscopic Studies on Aqueous Gels. Chemistry and Physics of Lipids 1985, 37 (3), 227–240.